dicembre 18, 2020 - Bosch
Bosch's rapid coronavirus test delivers results for positive samples in less than 30 minutes
Comunicato Stampa disponibile solo in lingua originale.
Cloud-based software update means a faster PCR test for SARS-CoV-2
- Improved software for Vivalytic: turnaround time for positive SARS-CoV-2 samples reduced to under 30 minutes.
- Expansion of production: Launch of two new productions lines in December.
- New jobs created: 80 associates – some from Bosch’s mobility division – were newly hired this year.
- Update from the cloud: Vivalytic analysis devices are updated over the internet.
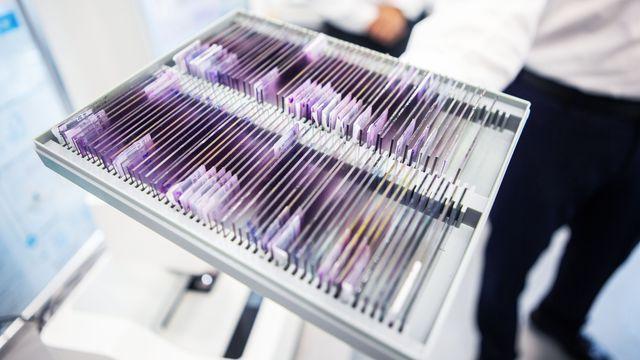
Stuttgart, Germany – Thanks to improved software for the Vivalytic analysis device, the Bosch rapid coronavirus test, which uses polymerase chain reactions (PCR), now delivers its results even faster. By applying optimized evaluation strategies, the Vivalytic analysis device can detect a positive sample through its high viral load in less than 30 minutes, right where the sample is collected. “In the fight to contain the coronavirus pandemic, speed is of the essence. With Vivalytic, we are delivering cutting-edge medical technology. Our IT and software expertise have helped to make the Bosch Corona test for positive samples even faster – all within a short period of time,” says Dr. Volkmar Denner, chairman of the board of management of Robert Bosch GmbH. This modified product is part of a research and development project relating specifically to Covid-19, funded by the German Federal Ministry of Education and Research (BMBF) to the tune of 4.97 million euros. The project’s aim is to expand the options for detecting acute infections of the coronavirus, looking particularly at on-the-spot testing in which the sample is analyzed in a fully automatic PCR process, as this offers rapid yet reliable results.
"In the fight to contain the coronavirus pandemic, speed is of the essence. With Vivalytic, we are delivering cutting-edge medical technology. Our IT and software expertise have helped to make the Bosch Corona test for positive samples even faster – all within a short period of time.”
Dr. Volkmar Denner, chairman of the board of management of Robert Bosch GmbH
The turnaround time for samples negative for SARS-CoV-2 is still 39 minutes. PCR tests are considered the gold standard, and Bosch’s rapid coronavirus tests have a sensitivity of 98 percent and a specificity of 100 percent. They are already available in 26 countries, mostly in Europe. “To meet the high demand, we launched two new production lines at our headquarters in Waiblingen in December,” says Marc Meier, president of Bosch Healthcare Solutions. “In total, we were able to create more than 80 new jobs this year, some of which have been filled by associates from Bosch's mobility division,” Marc Meier continues.
Update from the cloud platform Vivasuite
The new update is available for the SARS-CoV-2 singleplex test and the SARS-CoV-2 pooled test. Updating the Vivalytic testing devices is simple and straightforward – all that is required is an internet connection to access the Bosch cloud platform Vivasuite. Developed in-house at Bosch, the Vivasuite cloud platform allows users to digitally manage and update all their Bosch Healthcare Solutions devices. This is also an advantage when Vivalytic devices are in use in the field. The platform meets the strictest security standards and data privacy is guaranteed at all times: for example, there is no remote access to Vivalytic devices, and no possibility of accessing patient data.


















































































 Inglese
Inglese  Condividi
Condividi Condividi via mail
Condividi via mail  Automotive
Automotive Sport
Sport Events
Events Art&Culture
Art&Culture Design
Design Fashion&Beauty
Fashion&Beauty Food&Hospitality
Food&Hospitality Tecnologia
Tecnologia Nautica
Nautica Racing
Racing Excellence
Excellence Corporate
Corporate OffBeat
OffBeat Green
Green Gift
Gift Pop
Pop Heritage
Heritage Entertainment
Entertainment Health & Wellness
Health & Wellness